PO 40: Describe how liming materials increase soil pH.
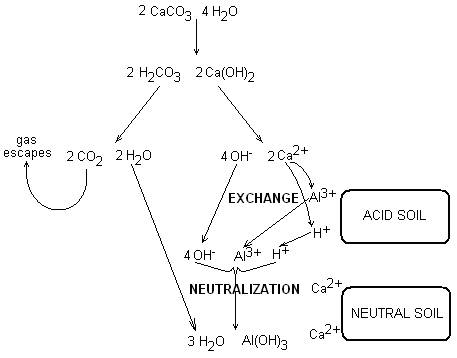
- Limestone is calcium carbonate and magnesium carbonate: CaCO3 and MgCO3
- The limestone dissolves in water to form carbonic acid (H2CO3) and calcium hydroxide (Ca(OH)2): CaCO3 + H2O ↔ H2CO3 + Ca(OH)2
- Carbonic acid is unstable and converts to carbon dioxide (CO2) and water; the CO2 gas escapes: H2CO3 ↔ CO2 + H2O
- The remaining calcium hydroxide dissociates: Ca(OH)2 ↔ Ca2+ + 2OH-
- The Ca2+ replaces 2H+ from the soil, increasing the soil base saturation
- The hydroxide anion (OH-) reacts with the soil acid cation (H+), forming water: OH- + H+ ↔ H2O
|
return to top | previous page | next page