Nutrient Management
Competency Area 5: Soil pH and Liming
|
pH is a soil characteristic that is affected by management, and is essential for optimal uptake of nutrients by plants. While the concentration of hydrogen ions (H+) is what determines a soil's pH, other factors such as soil type, fertilization, etc. influence the concentration of H+, and thus the pH. Liming soils to increase soil pH allows the plants to take up the proper nutrients. Many liming materials are available commercially, the speed and strength of which can be calculated using Fineness Values, Calcium Carbonate Equivalents, and Effective Neutralizing Value. These calculations are also essential to determining the rate of application. |
|
Soil pH is the negative log of the H+ ion concentration: pH = -log H+ = log 1/H+. The more H+ there is, the lower the pH and the greater the acidity.
Exchangeable acidity is a measure of the soil's ability to withstand a change in pH upon lime addition. The higher the exchangeable acidity of a soil, the more lime is needed for a particular pH change. |
|
Buffer pH is used to estimate a soil's exchangeable acidity; the degree of change in buffer pH is related to lime needs. Buffering occurs when an acid (for instance, H+) and a base (for instance, OH-) react and form a neutral product (in this case, H+ + OH- → H2O). Alkalinity is the term used to describe the amount of base in a soil when the pH is above 7. At higher alkalinity, there are fewer hydrogen ions (H+), and more hydroxyl ions (OH-). Basically, pH is a measure of active acidity, telling you whether you need lime or not. Buffer pH and exchangeable acidity measure the buffer capacity, telling you how much lime you need to add. There are several causes of soil acidity, including the leaching of basic cations (Ca2+, Mg2+, K+; leaving behind Al3+, which is acidic), crop uptake of basic cations and release of acids, decay of plant residues, acid rain, and the reaction of nitrogen fertilizer. There are many benefits of liming: it prevents the toxic effects of aluminum, increases availability of essential nutrients, supplies plant needs for Ca and Mg, improves soil conditions for microorganisms, increases effectiveness of some key herbicides, and improves soil texture. |
|
Nitrification or the conversion of ammonium N to nitrate N produces acidity: 2 NH4+ + 4 O2 → 2 NO3- + 4 H+ + H2O 2 H+ are produced for every N in the ammonium-N form (NH4+). This reaction occurs regardless of the source of the NH4+. This acidity is often the largest single source of acidity in fertilized agricultural soils. The net amount of acidity created when N fertilizer is applied depends on other reactions that occur with the fertilizer. The acidity created by different fertilizer materials is summarized below, as is the lb CaCO3 required to neutralize the acid. |
|
This information can be used to calculate the amount of lime required to neutralize a given application of fertilizer. For example:
The following reactions explain why there are differences in the amount of acidity per pound of N for the different N fertilizer materials.
|
|
Lime requirements increase with CEC, and a soil's CEC increases with organic matter content and clay content. Thus, clay soils with high organic matter content require more lime for a similar pH change than sandy soils with low organic matter. In other words, high CEC soils tend to be well-buffered, requiring more lime to change the pH; while sandy soils are poorly-buffered, requiring less lime per unit pH change. Also, because of the greater buffering, the soil pH will decrease slower on high CEC soils than on poorly-buffered sandy soils. In reduced-tillage systems, acidifying effects of N are concentrated at the soil surface. This is why two samples are required for pH testing: 0-1" and 0-6" (or 0-8").
|
|
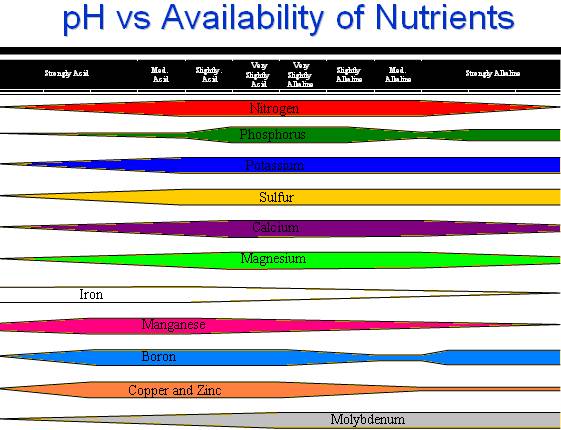
Soil pH affects nutrient availability by changing the form of the nutrient in the soil. Adjusting soil pH to a recommended value can increase the availability of important nutrients. Plants usually grow well at pH values above 5.5. Soil pH of 6.5 is usually considered optimum for nutrient availability. Lower pH increases the solubility of Al, Mn, and Fe, which are toxic to plants in excess. A critical effect of excess soluble Al is the slowing or stopping of root growth. Extreme pH values decrease the availability of most nutrients. Low pH reduces the availability of the macro- and secondary nutrients, while high pH reduces the availability of most micronutrients. Microbial activity may also be reduced or changed. |
Favorable pH ranges for common crops are listed in the table below.
|
|
Calcium Carbonate Equivalent (CCE) is the neutralizing value of a liming material compared to pure calcium carbonate. A CCE of 100% indicates that a material will neutralize the same amount of acidity per pound as pure calcium carbonate. |
|
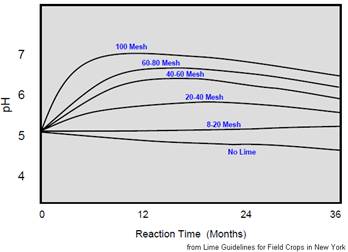
Fineness is related to how small the lime particles are. The finer a limestone is ground, the faster it will react in the soil. Fineness is reported as a particle size distribution usually as the percentage of the material that will pass 20, 60, and 100-mesh screens. An example of the size distribution for a typical good-quality limestone is: 95% passing 20 mesh; 60% passing 60 mesh; 50% passing 100 mesh. A distribution such as the one above is a good compromise between cost and practical agronomic effectiveness. Material larger than 20 mesh reacts too slow to be of much value as a liming material. All very fine, less than 100 mesh material will react quickly but it will be much more expensive and may be difficult to handle. Fineness does not increase the total neutralizing value of a limestone, just how fast it will react. |
|
Effective Neutralizing Value (ENV) or Effective CEE (ECCE) allows comparison of different liming materials, determined by the CCE x particle size distribution expressed as fineness. Limestones react at rates proportional to the surface area of the particles, and the surface area of the particles is related to the size of the lime particle. The CCE portion of the lime will react.
To determine the ENV:
|
Example:
The cost per ton of 100% ENV represents the cost for the quantity of material that will react with the soil in the first year, and allows cost comparison with other liming materials.
Soil test recommendations are based on limestone quality. Therefore, the recommendation must be adjusted for the quality of the limestone to be used. PA guidelines are based on CCE and fineness; NY guidelines are based on ENV.
|
Lime recommendations are given in 100% ENV. To convert to lime recommendation for a particular liming material, some calculations must be done.
|
|
In general, heavy metal availability is highest at low pH. Thus, liming can decrease heavy metal availability; at low pH these heavy metals could become toxic. Biosolids can contain heavy metals, while some biosolids might also be lime-stabilized, resulting in a pH increase upon application. |
|